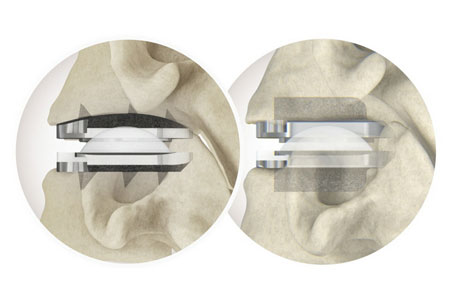
Centinel Spine®, LLC ("the Company"), the leading global medical device company focused exclusively on treating cervical and lumbar spinal disease with the most complete and clinically-proven total disc replacement (TDR) technology platform in the world (prodisc®), announced the completion of the 5,000th case in the U.S. with the prodisc C Vivo and prodisc C SK Cervical TDR System.
The prodisc C Vivo and prodisc C SK technologies are part of Centinel Spine's new Match-the-Disc™ System, made available in a limited release in Q4 2022. Over 600 surgeons have used the new system, confirming the benefits of intraoperatively selecting the appropriate implant for each patient's anatomy. Nearly 75% of the surgeon users are repeat users and, with the new system, the Company is significantly accelerating competitive conversions and increasing utilization within its user base. The 5,000 procedure milestone was achieved rapidly, taking just half the time needed to surpass the 2,500 procedure milestone in September 2023.
According to Dr. J. Alex Sielatycki, orthopedic spine surgeon with Steamboat Orthopedic & Spine Institute, Steamboat Springs, CO, "The prodisc cervical system is a phenomenal tool for the cervical arthroplasty surgeon. The wide range of implant options and customizability allows the surgeon to accommodate variations in endplate anatomy, bone quality, width, etc. with a single system. I can book any case with the prodisc cervical system and be confident that the right implant will be available when I call for it in the OR."
"The rapid uptake and repeat usage of the prodisc C Vivo and prodisc C SK Match-the-Disc system demonstrates the value optionality brings to surgeons," observes Centinel Spine CEO Steve Murray. "This new cervical total disc system is built on the prodisc technology platform that has been proven over the last three decades, delivering a unique combination of innovation and proven long-term clinical performance."
The prodisc portfolio is the most extensive TDR system in the world, offering both cervical and lumbar implants with anatomically-differentiated designs. Centinel Spine is the only company with FDA approval for both cervical and lumbar TDR systems. All of the prodisc cervical and lumbar devices incorporate prodisc CORE technology, the basis behind the predictable clinical outcomes of the prodisc platform after 30 years and over 250,000 implantations worldwide.*
For more information, please visit www.CentinelSpine.com