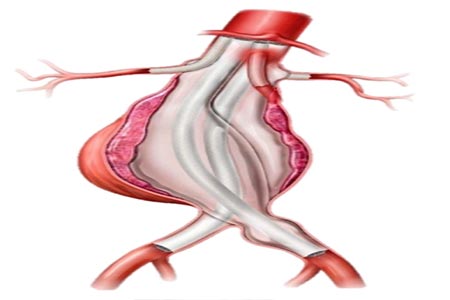
Endologix LLC, a leader in the treatment of vascular disease, the company’s ChEVAS™ (Chimney EndoVascular Aneurysm Sealing) System has been granted a Breakthrough Device Designation from the U.S.
Food and Drug Administration (FDA). The ChEVAS System is an investigational endovascular abdominal aortic aneurysm (AAA) sealing therapy designed to combine the Nellix® 3.5 endograft with parallel visceral stents to enable treatment of patients with juxtarenal, pararenal and suprarenal AAA.
The FDA Breakthrough Devices Program gives patients more timely access to medical devices that may provide for more effective treatment or diagnosis of life-threatening or irreversibly debilitating diseases or conditions. Breakthrough Devices receive priority review by FDA, and the program provides opportunities for early interaction with FDA personnel to expedite the review and approval process.
ChEVAS is currently being evaluated through the ChEVAS ONE IDE (Investigational Device Exemption) clinical study that is approved to enroll approximately 120 patients at up to 50 clinical sites worldwide. The national principal investigators of the ChEVAS ONE study are Francis Caputo, MD (Cleveland Clinic Foundation); William Jordan, MD (Emory University School of Medicine); Joseph Lombardi, MD (Cooper University Health Care) and William Quinones-Baldrich, MD (UCLA).
“The aneurysm sac sealing technology featured in the ChEVAS system is designed to reduce endoleaks, including gutter endoleaks, that are reported after endovascular treatment of complex aneurysms,” said James McKinsey, MD, the leading enroller in the ChEVAS ONE IDE study. “Our initial clinical results of this therapy have been promising in a challenging group of patients.”
Dr. McKinsey will present his initial experience with ChEVAS at the Eastern Vascular Society Annual Meeting on Sept. 26.
“The ChEVAS System represents an important therapy that provides an ‘off-the shelf’ treatment to an underserved patient population who have complex abdominal aortic aneurysms,” said Matt Thompson, MD, chief medical officer at Endologix. “We are delighted that the FDA has designated ChEVAS as a Breakthrough Device, as this will facilitate our ability to develop this technology and make it available to patients in an expedited fashion. The ChEVAS System joins the PQ Bypass DETOUR System as the two FDA designated Breakthrough Devices in our clinical investigational programs, which is reflective of our aspiration to provide innovative and disruptive technologies to address clinically relevant therapeutic gaps.”
For more information to visit http://www.endologix.com/
.
About Deerfield Management
Deerfield is an investment management firm committed to advancing healthcare through investment, information, and philanthropy.
For more information, please visit www.deerfield.com.