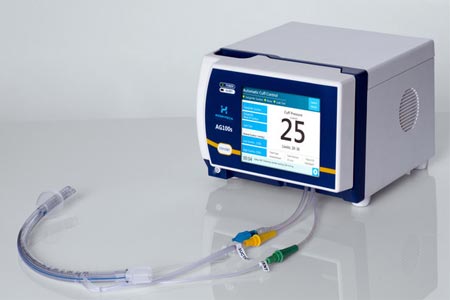
Following the AnapnoGuard™ airway management system's FDA market clearance at the end of 2018, Hospitech Respiration mentioned a new article was recently published by physicians from Mayo Clinic, FL, USA. It describes the successful use of the AnapnoGuard system in postoperative management of lung transplant patients.
The AnapnoGuard system (AG100s control unit and AG ETT) is a novel system which continuously monitors leaks around the endotracheal tube (ETT) cuff, automatically adjusts the cuff pressure to ensure sealing at minimal pressure, and evacuates subglottic secretions by simultaneous suction and rinsing.
This published data suggests that the AnapnoGuard system holds the potential to assist the care team in attenuating complications related to excessive cuff pressure, aspiration of subglottic secretions and endobronchial intubation. The data demonstrates quantitative removal of secretions and effective airway cuff pressure management.
Major risks and complications can occur following lung transplantation, with prolonged mechanical ventilation, increasing the risk of bacterial colonization and infections.
The presented data in this publication demonstrates the potential contribution of the AnapnoGuard system to:
- Compliance with airway management and VAP prevention guidelines
- Patient safety
- Reduction of complications which may result from excessive or insufficient ETT cuff pressure
- Reduction of lung contamination by effective evacuation of subglottic secretions
"This publication highlights the importance of taking steps towards reducing potential complications in mechanically ventilated patients, especially in high risk patient populations, such as post lung transplant and post cardiac surgery patients. It further lends support that the AnapnoGuard system may contribute to successful outcomes in prolonged ventilated patients. The company looks forward to generating further supporting data as the use of the AnapnoGuard system expands in the USA and Europe," stated Dr. William Denman, MD FRCA, Medical Director Anesthesiologist, Massachusetts General Hospital, Harvard Medical School.
For more details, visit: https://www.hospitech.co.il