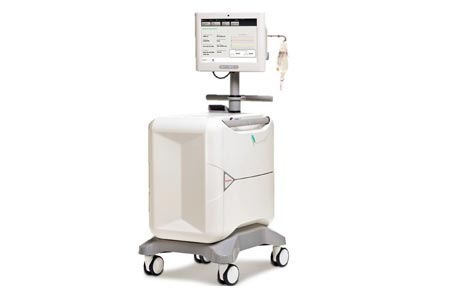
Jubilant DraxImage Inc. ("DraxImage"), a wholly owned subsidiary of Jubilant Pharma Limited, is pleased to announce the receipt of the CE Mark certificate for its state-of-the-art RUBY Rubidium Elution System (RbES) and the proprietary RUBY consumable accessories dedicated for use with their RUBY-FILL® (Rubidium Rb82 Generator).
This certificate allows the device to be distributed into the European Economic Area (EEA).
The RUBY-FILL® Generator and the RUBY Rubidium Elution System provide the latest technology in Positron Emission Tomography (PET) Myocardial Imaging. RUBY-FILL® is a closed system used to produce personalized patient doses of rubidium (Rb82) chloride injection for intravenous use. Rb82 chloride injection is a radioactive diagnostic agent indicated for PET imaging of the myocardium under rest or pharmacologic stress conditions to evaluate regional myocardial perfusion in adult patients with suspected or existing coronary artery disease.
About RUBY-FILL® :
INDICATION (US): RUBY-FILL is a closed system used to produce rubidium (Rb82) chloride injection for intravenous use. Rubidium (Rb82) chloride injection is a radioactive diagnostic agent indicated for Positron Emission Tomography (PET) imaging of the myocardium under rest or pharmacologic stress conditions to evaluate regional myocardial perfusion in adult patients with suspected or existing coronary artery disease.
New Important Safety Information - April 2019 (US)
WARNING: HIGH-LEVEL RADIATION EXPOSURE WITH USE OF INCORRECT ELUENT AND FAILURE TO FOLLOW QUALITY CONTROL TESTING PROCEDURE
Please see full prescribing information for complete boxed warning.
High-Level Radiation Exposure with Use of Incorrect Eluent
Using the incorrect eluent can cause high Strontium (Sr 82) and (Sr 85) breakthrough levels (5.1)
- Use only additive-free 0.9% Sodium Chloride Injection USP to elute the generator (2.5)
- Immediately stop the patient infusion and discontinue the use of the affected RUBY-FILL generator if the incorrect solution is used to elute the generator (4)
- Evaluate the patient's radiation absorbed dose and monitor for the effects of radiation to critical organs such as bone marrow (2.9)
Excess Radiation Exposure with Failure to Follow the Quality Control Testing Procedure
Excess radiation exposure occurs when the levels of Sr 82 or Sr 85 in the Rubidium Rb 82 Chloride injection exceed specific limits. (5.2)
- Strictly adhere to the generator quality control testing procedure. (2.6)
- Stop using the generator if it reaches any of its Expiration Limit. (2.7)
Jubilant Pharma Senior Vice-President of Medical and Chief Medical Officer Dr. Norman LaFrance stated, "Jubilant DraxImage is extremely excited about the CE Mark certificate, which will provide expanded access of this next-generation PET product into the EEA. With its advanced weight-based dosing accuracy and infusion options, RUBY-FILL will enhance the way patients with known or suspected coronary artery disease are both diagnosed and managed."
"This approval is another landmark event for Jubilant DraxImage, supporting its ongoing commitment to the global expansion and utility of nuclear medicine and PET imaging. Our goal is to grow SPECT and PET imaging globally by providing products that enable physicians to deliver high-quality diagnostic studies as part of our mission of Improving Lives Through Nuclear Medicine," stated Pramod Yadav, CEO Jubilant Pharma LTD.
Please visit www.draximage.com for Full Prescribing Information. People are encouraged to report negative side effects of prescription drugs.