Masimo (NASDAQ: MASI) today announced the findings of an abstract recently presented at ANESTHESIOLOGY® 2020, the Annual Meeting of the American Society of Anesthesiologists (ASA).
In this independent, retrospective study, researchers investigated whether Masimo ORi™ (Oxygen Reserve Index) could predict whether children with obstructive sleep apnea syndrome (OSAS) undergoing tonsillectomy required postoperative oxygen therapy.
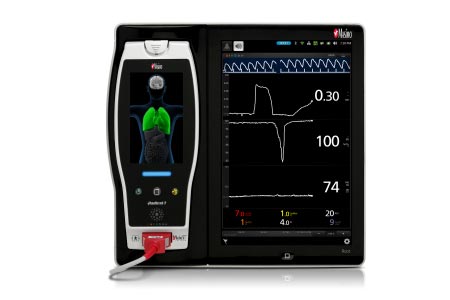
ORi, available outside the U.S., is a noninvasive and continuous parameter intended to provide insight into a patient’s oxygen status during moderate hyperoxia. Enabled by the multi-wavelength rainbow® Pulse CO-Oximetry platform, ORi is provided alongside oxygen saturation (SpO2), a clinically proven Masimo SET® pulse oximetry measurement.
Dr. Yoshimi Inagaki and colleagues at Tottori University in Yonago, Japan sought to determine whether Masimo ORi could serve as a useful predictor of the need for postoperative oxygen therapy (POT). They enrolled 45 pediatric patients with OSAS, ranging from 7 to 120 months, who were anesthetized with sevoflurane and monitored with ORi while undergoing tonsillectomy.
Of the 45 patients, 16 required POT. For those 16, the mean lowest ORi and SpO2 values were 0.28 and 93%, respectively. For the remaining patients, who did not receive POT, the mean lowest values were 0.64 and 97%, respectively. The researchers calculated sensitivity and specificity for ORi predicting when POT would not be needed of 0.815 (95% confidence interval 0.5435 – 0.9595) and 0.9310 (95% confidence interval 0.7723 – 0.9915), respectively.
The researchers concluded that ORi is “likely to become an index of POT in pediatric patients with OSAS.” They also noted, “In children with OSAS, [the] requirement of POT following tonsillectomy and adenoidectomy is probably able to be predicted on the basis of the results of this retrospective cohort study.”
ORi has not received FDA 510(k) clearance and is not available for sale in the United States.
for more details, visit: www.masimo.com