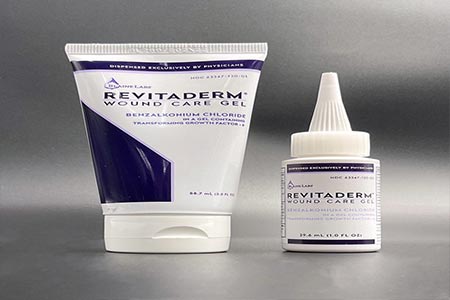
The product is used as a skin wound antimicrobial and is packaged in a 1.0 ounce bottle and a 3.0 ounce tube with each labeled as "RevitaDerm Wound Care Gel" with a Drug Facts label on the back.
The affected RevitaDerm Wound Care Gel lot is BL 2844 with an expiration date of 02/19/2023. The product can be identified by its name on the front of either the 1.0 ounce bottle or the 3.0 ounce tube, and the 1.0 ounce volume has the witch-hat dispensing cap.
The RevitaDerm Wound Care Gel Product was distributed to 61 physician clinics in 17 states in the United States in the year 2021.
Risk Statement: Patients who apply the contaminated product to a wound could develop a skin and soft tissue infection which could lead to serious complications. For non-immunocompromised patients these infections are expected to be less severe and responsive to treatment.
However, for the immunocompromised patients and preterm neonates, Bacillus cereus can cause life-threatening, invasive infections including wound and blood infections, sepsis, pneumonia, and meningitis. To date, Blaine Labs Company has not received any complaints or reports of adverse events related to this lot of 1.0 ounce bottle or 3.0 ounce tube of RevitaDerm Wound Care Gel.
Blaine Labs is notifying its physician clients by email, regular mail and by phone, and is arranging for the return of undispensed 1.0 ounce bottles and 3.0 ounce tubes from lot BL 2844.
Patients who have the RevitaDerm Wound Care Gel 1.0 ounce bottle or 3.0 ounce tubes, which are being recalled, should stop using the product and return unused product to the dispensing physician.
Adverse reactions or quality problems experienced with the use of this product may be reported to the FDA's MedWatch Adverse Event Reporting program either online, by regular mail or by fax.