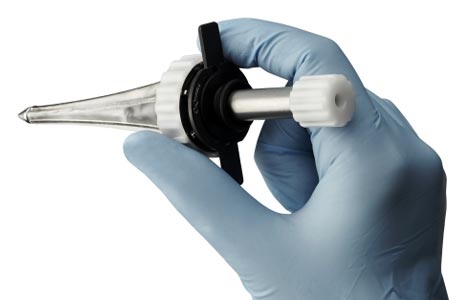
Minnetronix Medical has been announced that it has received FDA clearance for its first platform product: the MindsEye™ Expandable Port for neurosurgical procedures.
This clearance represents an expansion of the company’s traditional offerings to include market-ready platforms.
The MindsEye Port is the first minimally invasive, expandable port designed for use in the treatment of stroke, cancer, and other conditions. The device’s dynamic retraction creates a custom-sized channel allowing surgeons to reach target areas deep within the brain. “This is next-generation deep brain access technology,” said Dr. Mario Zuccarello, professor of Neurosurgery, University of Cincinnati Medical Center. “Minimizing invasiveness as much as possible is important to respect the eloquence of brain tissue. The MindsEye Expandable Port allows surgeons to work without unnecessary distractions, which ultimately improves quality of life for the patient.”
“Given its unique features, the port has already attracted interest among neurosurgeons and potential distribution partners. They’re excited to get their hands on it,” says Matt Adams, vice president and general manager at Minnetronix Medical. “The MindsEye Port is an exciting example of how Minnetronix Medical is expanding its offerings by developing market-ready products.” He adds that the port represents an entire platform for the company, as it has additional applications and complimentary product opportunities in the neuro market and beyond.
For more details, visit: www.minnetronixneuro.com.