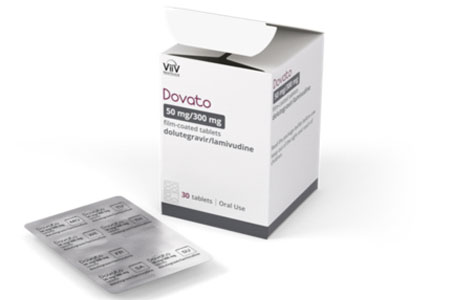
ViiV Healthcare, the global specialist HIV company majority owned by GSK, with Pfizer and Shionogi as shareholders, announced Dovato (dolutegravir/ lamivudine) is now available in a blister pack in the U.S.
Dovato is approved as a complete regimen to treat HIV-1 infection in adults with no antiretroviral (ARV) treatment history or to replace the current ARV regimen in those who are virologically suppressed (HIV-1 RNA <50 copies/mL) on a stable ARV regimen with no history of treatment failure and no known resistance to any component of Dovato.
Lynn Baxter, Head of North America at ViiV Healthcare, said: “The Dovato blister pack is designed to help address some of the challenges we hear from the HIV community, which include stigma and convenience, and offers a discreet package which may fit more seamlessly into people’s daily routines. Everyone has different experiences and preferences when it comes to their HIV treatment and, at ViiV Healthcare, we are pleased to offer a variety of treatment and packaging options that help suit the needs of people living with HIV.”
The Dovato blister pack is a monthly 30-count box containing five sheets of tablets. Created based on insights from the HIV community, each sheet is about the size of a credit card and perforated, and designed to be small, discreet, and not bulky. The sheets allow people living with HIV to view the number of pills left and track their doses.
The FDA approved the Dovato blister pack on November 3, 2023. ViiV Healthcare plans to offer the Dovato blister pack in some European markets in 2024. ViiV Healthcare will continue to offer Dovato in the 30-count pill bottle.
About Dovato (dolutegravir/lamivudine)
Dovato is a once-daily, single-pill, 2-drug regimen (2DR) that combines the integrase strand transfer inhibitor (INSTI) dolutegravir with the nucleoside reverse transcriptase inhibitor (NRTI) lamivudine.
Dovato is approved in the U.S., Europe, Japan, Australia and other countries worldwide.
Trademarks are owned by or licensed to the ViiV Healthcare group of companies.
About GSK
GSK is a global biopharma company with a purpose to unite science, technology, and talent to get ahead of disease together. Find out more at gsk.com.